Benefits and Risks of Participating in a Clinical Trial
Clinical trials are the backbone of medical progress. They allow researchers to test new treatments, therapies, and procedures to determine whether they are safe and effective for broader use. By participating in a clinical study, you’re not only gaining early access to cutting-edge medical care, you’re actively contributing to discoveries that may benefit millions in the future.
This article will help you understand the pros and cons, financial incentives and safety measures taken during these trials, so you can make smart choices. Whether you want to help medical research or earn extra money, knowing all about clinical trials is important.
Key Takeaways
- Understand the benefits and risks of participating in clinical trials.
- Evaluate personal motivations before joining a paid study.
- Consider the pros and cons of participating in paid studies.
- Be aware of the financial incentives and how they work.
- Learn about the informed consent process to protect your rights.
Introduction to Paid Studies
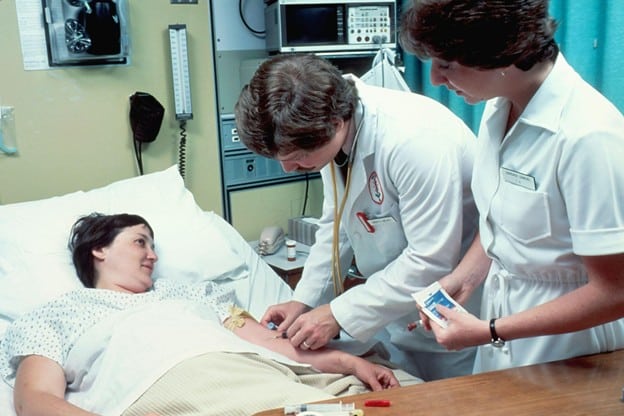
Clinical trials play a vital role in advancing medical science. They are designed to evaluate new treatments, medications, or procedures to determine their safety and effectiveness. By participating in these studies, you become an essential part of the discovery process contributing to innovations that may improve or even save lives in the future.
With over 515,000 registered clinical trials globally, opportunities to contribute to medical research are more accessible than ever. Clinical research gives qualified participants the opportunity to access treatments that are not yet available to the general public. For individuals managing specific health conditions, this can provide hope, access to expert medical oversight, and in some cases, early therapeutic options.
The Appeal of Participation
People choose to take part in clinical trials for a variety of personal and altruistic reasons. Some are motivated by the desire to improve their own health or explore alternatives when standard treatments haven’t worked. Others want to contribute to science and help future patients benefit from more effective care.
In addition to playing a role in medical advancement, participants often receive enhanced medical attention, including regular check-ups, monitoring, and diagnostic tests at no cost. This added care can improve overall healthcare experiences, making clinical trials an appealing option both medically and financially.
Recognizing the purpose and potential advantages of paid clinical studies empowers you to make an informed decision about applying—and to contribute to something greater than yourself.
Pros and Cons of Participating in Paid Studies
Joining a paid research study is a decision that can be both rewarding and impactful, but like any commitment, it’s important to weigh the benefits against the potential drawbacks. Understanding both sides will help you make an informed choice that aligns with your goals, values, and comfort level.
Potential Benefits of Participation
Participating in paid studies can offer a number of valuable advantages:
- Access to Innovative Treatments
Clinical trials and healthcare studies may offer early access to new medications, therapies, or devices before they’re widely available. - Personalized Healthcare
Many studies are condition-specific, meaning participants often receive specialized medical evaluations, care, or monitoring tailored to their health profile. - Contribution to Public Health
Your participation plays a crucial role in advancing science and improving future treatments for others. - Financial Compensation
Paid research studies often offer generous rewards, especially for in-depth or time-intensive participation. - Flexible Opportunities
From short online surveys to long-term clinical trials, you can choose studies that match your schedule and comfort level.
Possible Risks and Disadvantages
While there are benefits, there are also risks and considerations to keep in mind:
- Potential Side Effects
Especially in medical trials, new treatments may have unknown side effects or health risks. That’s why clinical trials include informed consent and regular monitoring. While participation contributes to scientific advancement, it’s noteworthy that only about 14% of investigational drugs successfully navigate the clinical trial process to gain FDA approval. - Time Commitment
Some studies require multiple visits, extended participation, or specific scheduling that can interfere with work, school, or family responsibilities. Depending on the phase, clinical trials can span from several months to multiple years, with Phase III studies potentially lasting up to five years. - Privacy Concerns
Sharing personal or medical information—even with reputable organizations—always carries some risk. Make sure you understand how your data will be used and protected. - Eligibility and Screening
Not everyone qualifies for every study. Screeners can be detailed, and rejection is common, especially in medical or high-paying opportunities.
By understanding both the advantages and the limitations, you can approach paid research opportunities with confidence and realistic expectations. The key is to choose studies that align with your goals and values and to partner only with trusted, transparent platforms that respect your time, safety, and privacy.
Financial Incentives for Participation
When you think about joining clinical trials, knowing about financial incentives is key. Money matters a lot when you decide to join. It’s important to understand how payments work and what they mean for your experience.
Understanding Payment Structures
Financial rewards in clinical trials can vary a lot. They depend on the type of study and how much time you need to give. Participants in Phase I trials typically receive compensation ranging from $10 to $20 per hour, reflecting the time and commitment required. Here are some common ways you might get paid:
- Flat fees for participation, which give you a set amount of money for finishing the study.
- Hourly rates that pay you for the time you spend during the trial.
- Reimbursement for travel expenses, making it easier to get to study visits.
Knowing about these payment options helps you see if they fit your needs and goals. The money offered can really affect how much you want to join a trial.
Reimbursement and Ethical Considerations
Ethics are very important when it comes to money in clinical trials. Researchers must make sure money doesn’t make people join without their free will. Being open about money is crucial to keeping trust and ethics high.
When it comes to paying for travel and other costs, think about how it makes participants feel. They should feel valued and respected, without losing their freedom to choose.
Informed Consent in Clinical Trials
Informed consent is important in clinical trials. It makes sure you know what you’re getting into. You get a consent document that explains the study’s details. It’s all about being clear and open, so you can decide wisely.
The Process of Informed Consent
The informed consent process starts with a talk with the research team. They’ll cover the study’s purpose, what you’ll do, and how long it will last. You’ll hear about:
- What role you’ll play in the study
- Any risks involved
- The benefits you might get
- How your data will be kept private
It’s important to ask questions during this time. The team should be ready to answer any concerns you have.
Key Information Participants Should Know
Your consent document is packed with important details. It should have:
| Aspect | Description |
| Study Overview | A brief on the research goals and how it’s done |
| Risks | Info on possible side effects or problems |
| Benefits | What good things might come out of it for you and others |
| Compensation | Details on any payments or reimbursements |
| Withdrawal Rights | Your right to leave the study anytime without trouble |
Knowing these things helps you fully participate in the trial. It’s not just about protecting your rights. It’s also about feeling sure about what you’re doing.
Safety Measures in Clinical Research
Keeping participants safe is key in clinical research. Many safety steps are taken from the start. This includes the important role of Institutional Review Boards (IRBs) and ongoing checks to keep the research safe.
The Role of Institutional Review Boards
Institutional Review Boards (IRBs) check studies before they start. They look at the risks and benefits to make sure safety comes first. They review the study plans, consent forms, and how people are invited to join.
IRBs are vital in protecting people in studies. They must approve a study before it can start.
Monitoring Participant Safety
After IRBs give their okay, keeping an eye on safety is ongoing. Data and safety monitoring boards watch over this. They check the data collected during the study for any safety issues.
These strong safety steps help researchers quickly deal with any problems. This keeps the focus on the safety and well-being of the participants.
Conclusion
Paid clinical studies offer more than just financial rewards; they provide an opportunity to better care, greater understanding, and real contributions to medical breakthroughs. Whether you’re motivated by the possibility of improving your own health or by the chance to help others, your participation matters.
By joining a trial, you help shape the future of medicine while gaining access to new treatments and expert monitoring. With careful consideration and by working with reputable research organizations, you can turn this opportunity into a meaningful experience, both for yourself and for generations to come.
FAQ
What are clinical trials?
Clinical trials test new treatments and understand diseases. They help develop better therapies. These studies are key to improving patient care.
What are the potential benefits of participating in a clinical trial?
You might get access to new treatments not yet public. You could also get personalized healthcare. Plus, you help society by improving health.
What risks should I consider before participating in a clinical trial?
Risks include side effects from new treatments. You might have to see doctors more often. There’s also a risk of losing privacy about your health.
How are participants compensated for their involvement in clinical trials?
Compensation varies and can include fees or travel reimbursement. But, make sure money doesn’t sway your decision to join.
What is informed consent in the context of clinical trials?
Informed consent means you know all about the study. This includes risks and benefits. It helps you make a smart choice.
How is participant safety monitored during clinical trials?
Safety checks are done by IRBs and data monitoring boards. They look at risks and benefits. They make sure you’re safe.
Can I withdraw from a clinical trial once I’ve agreed to participate?
Yes, you can leave the study anytime. You won’t face any penalties. It’s your choice if you feel uncomfortable or if the study doesn’t fit your goals.
What should I do if I have more questions about participating in a clinical trial?
Talk to your doctor or the research team. They can clear up any doubts. This helps you decide if joining a trial is right for you.